Methodology overview: Two Companies, Two Pillars
From Algorithm to Approval: Our methodology integrates cutting-edge AI algorithms to pinpoint potential drugs, followed by rigorous lab optimization and preclinical testing for safety and efficacy. This foundation sets the stage for advancing successful candidates through the critical stages of clinical trials, ensuring a streamlined path from discovery to Phase 3 clinical validation.
- Algorithmic Innovation: Our journey begins with our proprietary algorithm, a powerful tool designed to identify promising lead compounds with unprecedented speed and accuracy. Utilizing complex data analysis and predictive modeling, we sift through vast chemical libraries to uncover potential therapeutic agents, setting the stage for real-world applications.
- Laboratory Development and Preclinical Assessment: Transitioning from digital predictions to tangible results, selected compounds undergo rigorous chemical modification and optimization in our state-of-the-art laboratories. This phase is crucial for enhancing drug efficacy and reducing potential toxicity. Comprehensive toxicity screenings and preclinical studies are conducted to ensure safety and determine the optimal dosage, meticulously paving the way from laboratory breakthroughs to ready-for-human trials candidates.
- Clinical Trials and Validation: The final leap towards medical innovation is marked by our entry into phase 3 clinical trials, where the safety and effectiveness of our compounds are tested in human subjects. This critical stage validates our drug candidates' therapeutic potential, demonstrating their ability to address unmet medical needs and bringing them one step closer to regulatory approval and clinical use. Through this methodical progression, we bridge the gap between algorithmic discovery and the delivery of life-changing treatments to patients worldwide.
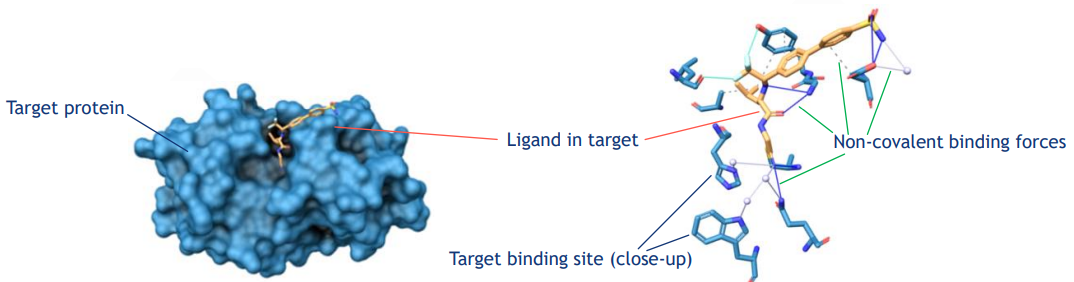
1. Use of atomic-level Biological Target Information
- Proteins: amino acid sequence, 3D structure, physico-chemical properties of binding sites
- Nucleic acids : nucleotide sequence, 3D structure, genetic context (regulatory, coding, non-coding etc.)
2. Abstraction of Target - Ligand interaction
- Standardised mathematical description
- Unique, structure-invariant 3D fingerprint
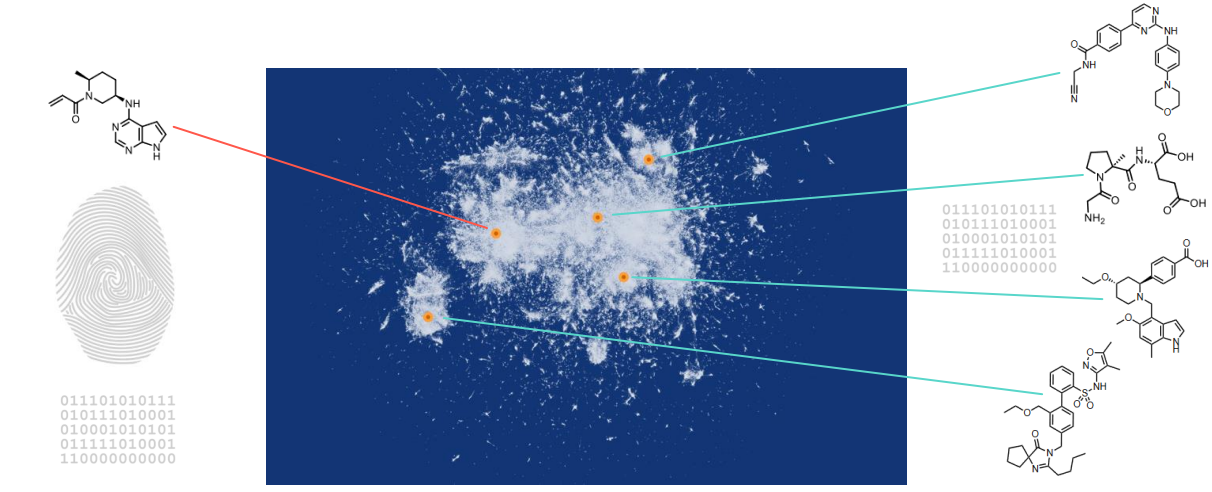
3. Analysis of 3D fingerprints
- Chemical space with 100 million+ theoretical and existing compounds (small molecules)
4. Clinical trial development
- Pre-clinical
- Clinical phase 1-2
- In collbration with ABX-CRO advanced pharmaceutical services Forschungsgesellschaft, Germany.
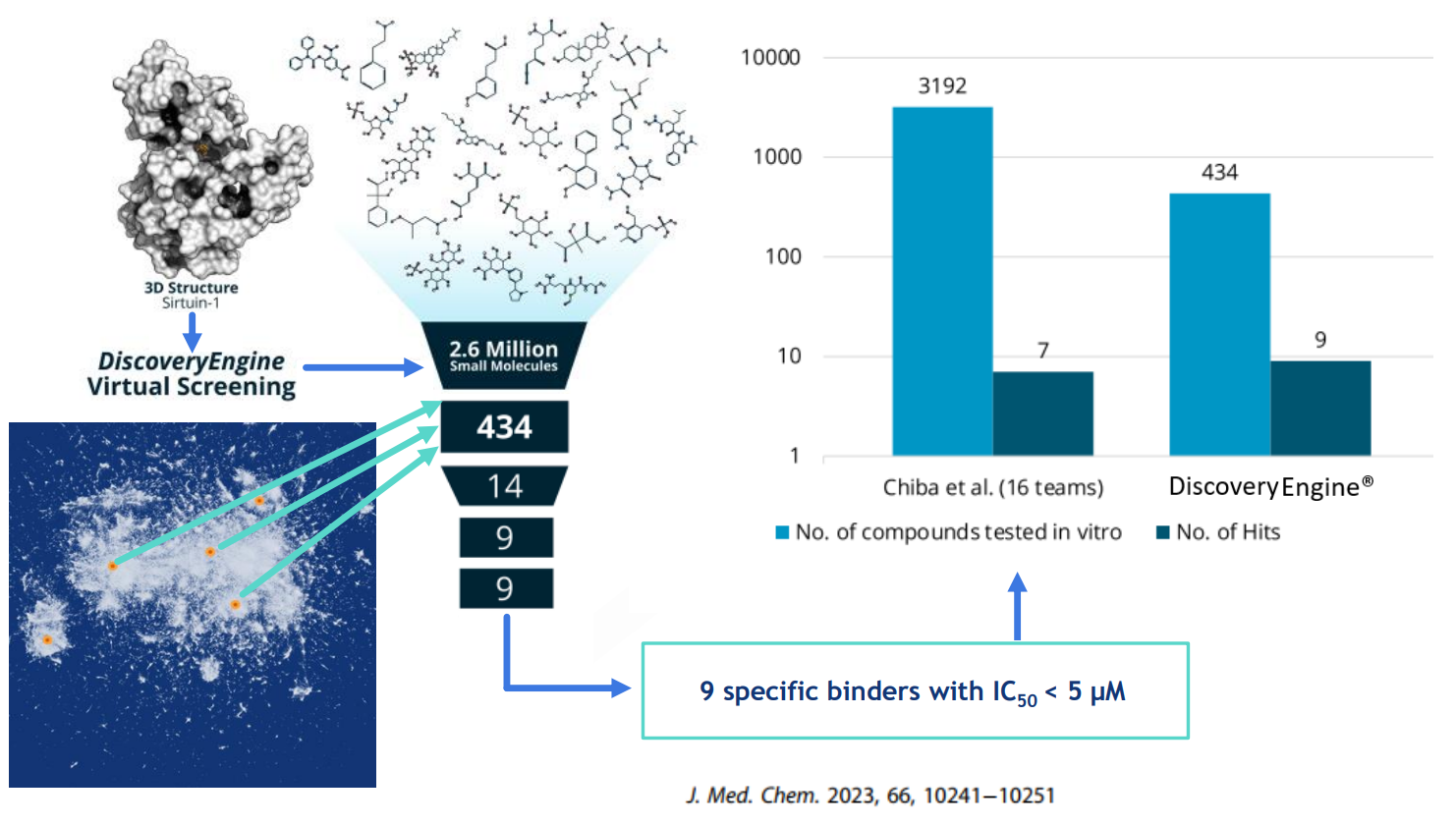
Performance benchmarking
- Outperforming competitive in silico methodologies quantitatively (hit rate) and qualitatively (affinity) by a Factor of 10x
Collaboration partners
- PharmAI Discovery and its daughter company PharmAI has successful collaboration projects with more than 20 prominent actors within the field.